Tomai lab. (FRIS)


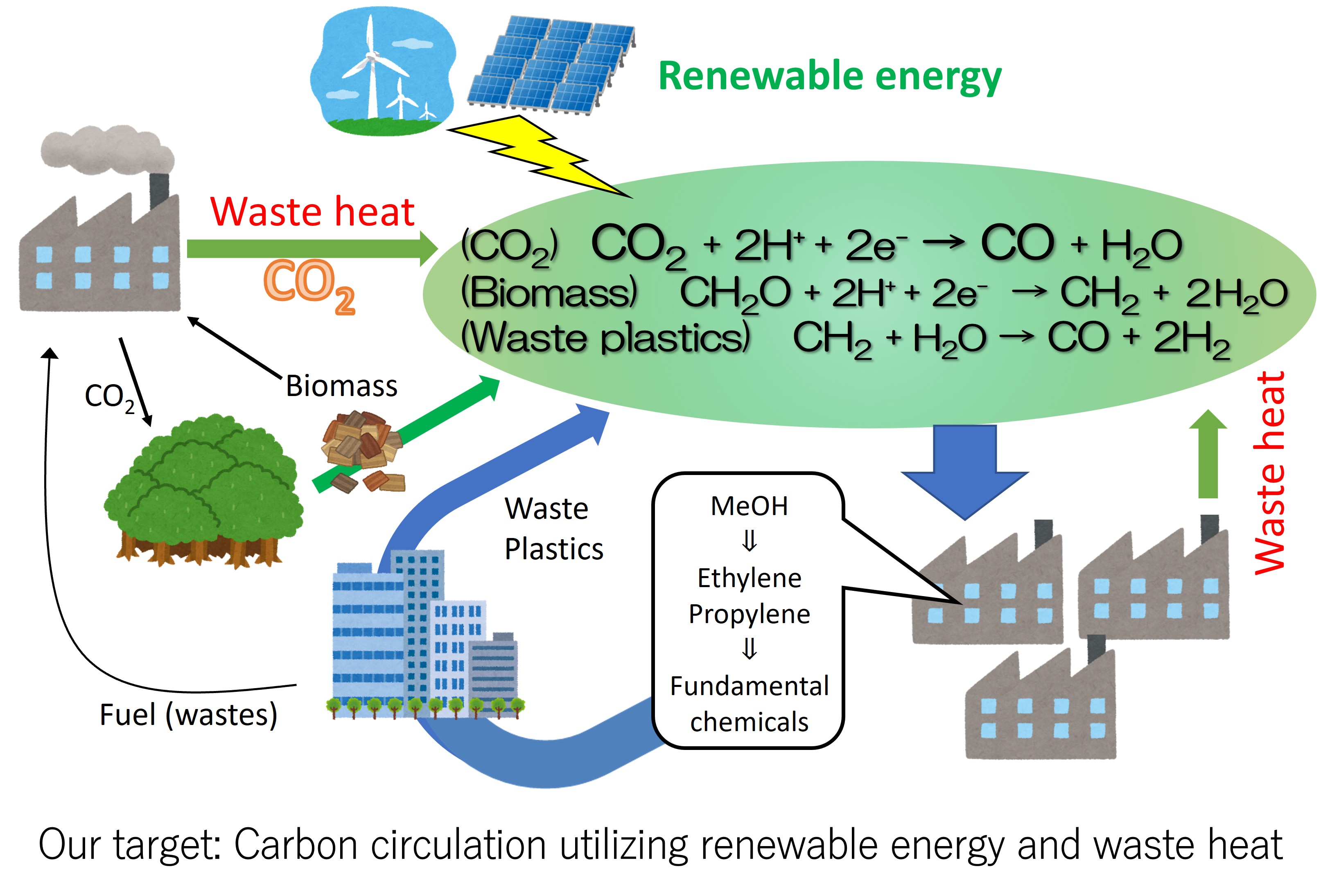
The high controllability of solvent properties is the advantage of supercritical fluid. For example, supercritical water is miscible with organic compounds. The unique characteristics of supercritical fluids enable the synthesis of unique nanomaterials that cannot be synthesized under conventional synthesis process. Our research group aims to develop innovative chemical process driven by low-temperature waste heat and renewable energy by designing unique nanomaterial. We are working on improving process/device by precisely controlling multi-scale hierarchical structure of nanomaterial from the composition and crystalline structure to the dispersion/aggregation (porous) structure. Moreover, we are developing electrochemical materialenergy conversion systems using supercritical and subcritical fluids as reaction fields by focusing on their unique properties, specifically carbon dioxide and water electrolysis. Through our research efforts, we aim to establish scientific principles for high-temperature, highpressure electrochemical energy conversion systems and contribute to realizing a sustainable society.